Plating with good corrosion resistance can be classified into galvanization using the action of sacrificial anti-corrosion (zinc-plating to iron base) and plating with good corrosion resistance of itself, which is represented by blik (tin-plated iron base). The latter plating excelling in corrosion resistance includes noble metal plating and electroless nickel plating having excellent chemical resistance.
| Type of plating | Characteristic value | Uses |
|---|---|---|
| Sacrificial anti-corrosive plating (primary plating or material is electrochemically protected from corrosion by the upper-layer plating film oxidizing slowly) | - Zinc plating and zinc-based plating preventing steel materials from rusting (See zinc and zinc alloy plating.) - Two-layer nickel plating Potential difference by the difference of sulfur content in the Ni film based on the principle of sacrificial anti-corrosion is used. The sulfur content in the lower layer of semigloss nickel is 0.005% and that in the upper layer of bright nickel is 0.05%. The plating thickness is approximately 20 – 30μm in total (semigloss:bright = 6:4). The corrosion resistance of three-layer nickel plating by sandwiching bright nickel containing 0.1 – 0.2% sulfur between the two layers of plating is further improved. |
Various metal products |
| Plating with good corrosion resistance in plating films themselves | - Plating with noble metals small in ionization tendency, such as gold, platinum, rhodium, and palladium. - Chemical-resistant electroless nickel plating, chromium plating, nickel-tungsten alloy plating, etc. The high corrosion resistance of nickel-based alloy plating originates in the passivation phenomenon of films. |
Chemical industry equipment |
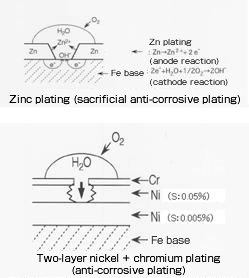
Types of plating