When the chromium content in steel exceeds 12%, the corrosion resistance improves dramatically. From this, iron-chromium alloys containing 12% or more chromium is called stainless steel. The corrosion resistance of this stainless steel originates in the ultrathin chromium oxide film (passive film) formed on the most superficial layer. From the perspective of the adhesion property of plating, how to remove this passive film is a factor.
| Classification | Main ingredient | Metallographic structure (crystalline structure) |
Magnetism | Uses |
|---|---|---|---|---|
| Cr-based stainless steel | 13%Cr (SUS410-based) | Martensite (body-centered cubic structure) | Strongly magnetic | All sorts of industries |
| 14%Cr (SUS430-based) | Ferrite (body-centered cubic structure) | Strongly magnetic | ||
| Cr-Ni-based stainless steel | 25%Cr (SUS329-based) | Austenite/ferrite | Magnetic | |
| 18%Cr-8%Ni (SUS304-based) | Austenite (face-centered cubic structure) | Non-magnetic |
- Others: Austenite/ferrite-based (SUS329-based): High resistance to chlorine.
Precipitation hardened (SUS630-based): High strength.
- Note: Even if stainless steel is non-magnetic, only the superficial layer is magnetized when subjected to strong cold working.
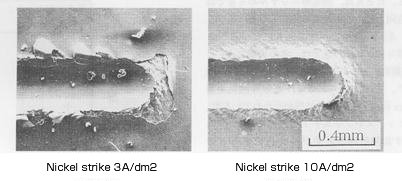
Appearance of electroless nickel plating
on stainless steel after adhesion test
Types of plating