Electrolysis of water produces hydrogen (cathode) and oxygen (anode) by giving electrical energy. Contrarily, water is generated and also electrical energy can be taken out by the reaction of hydrogen on the cathode side and oxygen on the anode side. This is the principle of fuel cells.
| Type | Features | Characteristic value |
|---|---|---|
| Phosphoric acid (PAFC) |
- Anode (fuel electrode side): Carbon material Fuel: Hydrogen, methanol, natural gas - Electrolyte: High-concentration phosphoric acid Charge carrier: Hydrogen ion - Cathode (air electrode side): Carbon material - Catalyst: Platinum or platinum ruthenium |
Low-temperature type Operating temperature: 160 – 210°C Uses: Dispersed power systems |
| Molten carbonate (MCFC) |
- Anode (fuel electrode side): Nickel Fuel: Natural gas, methanol, naphtha, coal gasification gas - Electrolyte: Lithium - Potassium carbonate Charge carrier: Carbonate ion - Cathode (air electrode side): Nickel oxide |
High-temperature type Operating temperature: 600 – 700°C Uses: Dispersed power systems, high-efficiency power generation |
| Solid oxide (SOFC) |
- Anode (fuel electrode side): Nickel/stabilized zirconia Fuel: Natural gas, methanol, naphtha, coal gasification gas - Electrolyte: Yttria-stabilized zirconia (YSZ) Charge carrier: Oxide ion - Cathode (air electrode side): Lanthanum manganite |
High-temperature type Operating temperature: 900 – 1,000°C Uses: Dispersed power systems, high-efficiency power generation |
| Polymer electrolyte | - Anode (fuel electrode side): Platinum Fuel: Hydrogen, methanol, natural gas - Electrolyte: Hydrogen-ion exchange membrane Charge carrier: Hydrogen ion - Cathode (air electrode side): Platinum |
Low-temperature type Operating temperature: 80 – 100°C Uses: Automotive power supplies, household power supplies, mobile power supplies |
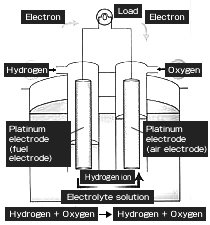
Schematic diagram of fuel cell
Types of plating