Nickel plating is used as various types of primary plating because it offers excellent corrosion resistance, thermal resistance, mechanical properties, and so on. It plays an active part across a wide range of areas, such as automobiles, home electrical appliances, and industrial machines, particularly as primary plating of chromium plating for decorative & corrosion-resistant purposes.
| Type of plating | Features (characteristic value) |
|---|---|
| Primary nickel two-layer plating | - Two-layer Ni plating (principle of sacrificial anti-corrosion) using the potential difference as a result of the difference in sulfur content in the Ni film offers excellent corrosion resistance. - The sulfur content in the lower layer of semigloss nickel is 0.005% and that in the upper layer of bright nickel is 0.05%. Natural electrode potential: (more noble) semigloss nickel > bright nickel (less noble) - The plating thickness is approximately 20 – 30μm in total. The film thickness ratio should preferably be semigloss:bright = 6:4. |
| Primary nickel three-layer plating | - Corrosion resistance is improved by sandwiching a plated layer having a higher sulfur content between the two layers of primary nickel plating. |
| Particulate dispersion composite nickel plating (trade name: DUR-Nickel Plating) |
- Insoluble inorganic fine particles (particle size approximately 0.02μm) are dispersed and co-deposited in the nickel plating film compositely. |
| Micropore chromium plating | - Although chromium plating itself is ordinary decorative chromium plating, when particulate dispersion composite plating is performed in the primary nickel plated layer, the particles are not chromium-plated, with the result that the chromium plating brings about micropores. The number of micropores is controlled in the range of 20,000 to 400,000 per square centimeter. Corrosion resistance is dramatically improved by the micropores dispersing corrosion current. |
| Microcrack chromium plating | - Chromium plating films are microcracked by adding a certain type of stress increasing agent to the chromium plating solution. Corrosion resistance is improved by the microcracks dispersing corrosion current as is the case with micropore chromium plating. |
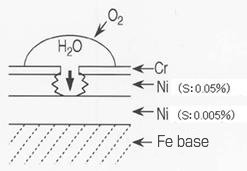
Schematic diagram of two-layer
nickel-chromium plating on iron base